Journal Article
,
Center for Human Nutrition, Washington University School of Medicine
,
St. Louis, MO 63110
,
USA
Office of Nursing Research, Goldfarb School of Nursing at Barnes-Jewish College
,
St. Louis, MO 63110
,
USA
Search for other works by this author on:
,
Center for Human Nutrition, Washington University School of Medicine
,
St. Louis, MO 63110
,
USA
Search for other works by this author on:
,
Center for Human Nutrition, Washington University School of Medicine
,
St. Louis, MO 63110
,
USA
Division of Nephrology, Endocrinology and Metabolism, Department of Internal Medicine, Keio University School of Medicine
,
Tokyo 160-0016
,
Japan
Search for other works by this author on:
Center for Human Nutrition, Washington University School of Medicine
,
St. Louis, MO 63110
,
USA
Sansum Diabetes Research Institute
,
Santa Barbara, CA 93105
,
USA
Correspondence: Samuel Klein, MD, Center for Human Nutrition, Washington University School of Medicine, 660 South Euclid Ave, Mail Stop 8031-0014-02, St Louis, MO 63110. Email: sklein@wustl.edu.
Search for other works by this author on:
Received:
13 December 2022
Editorial decision:
24 January 2023
Corrected and typeset:
24 February 2023
Published:
24 February 2023
Abstract
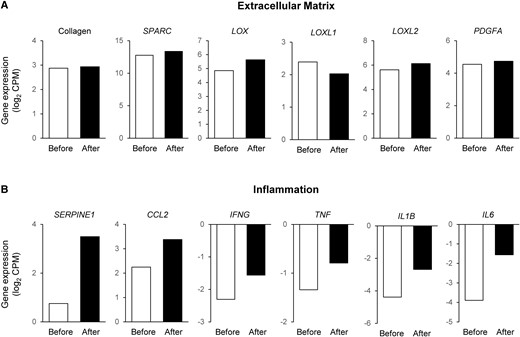
People with obesity who do not have the metabolic syndrome or components of the metabolic syndrome have been characterized as having metabolically healthy obesity (MHO). However, the existence of MHO has been questioned because people with MHO are at greater risk of developing diabetes and fatal cardiovascular disease than people who are lean and healthy. Here we report findings from a 25-year-old woman with rigorously defined MHO (normal oral glucose tolerance, insulin sensitivity [assessed using the hyperinsulinemic-euglycemic clamp procedure], plasma triglyceride, and intrahepatic triglyceride content) evaluated at baseline (body mass index, 37.7 kg/m2) and 5 years later, after a 32% (30.8 kg) increase in body mass (BMI, 49.6 kg/m2). Weight gain did not have adverse effects on fasting plasma glucose, oral glucose tolerance, β-cell function, insulin sensitivity, plasma triglyceride, intrahepatic triglyceride content, or carotid intima-media thickness. Adipose tissue expression of genes involved in extracellular matrix formation remained unchanged. Adipose tissue expression of several inflammation-related genes increased by more than 30%, but was not associated with a corresponding increase in plasma cytokine concentrations, with the exception of IL-6 and C-reactive protein. The present case study demonstrates that some people with obesity are resistant to the adverse cardiometabolic effects of excess adiposity and marked weight gain.
Obesity is typically associated with a variety of cardiometabolic comorbidities, including insulin resistance, atherogenic dyslipidemia, nonalcoholic fatty liver disease, prediabetes, and the metabolic syndrome [1]. However, some people with obesity do not have these complications and are considered “metabolically healthy” [2]. Even though the risk of all-cause mortality, type 2 diabetes, and coronary heart disease in people classified as having metabolically healthy obesity (MHO) is lower than those with metabolically unhealthy obesity (MUO), the risk of developing cardiometabolic comorbidities is still greater in people with MHO compared with people who are healthy and normal weight. In addition, many people with MHO convert to MUO over time [3]. As a result, it has been proposed that MHO does not really exist and all people with obesity are at increased risk for cardiometabolic diseases. A major limitation of these studies is the absence of a single, rigorous definition of MHO. More than 30 different definitions of MHO have been used in previous studies [4], and most criteria allow people to be classified as MHO even if they have evidence of metabolic abnormalities (eg, people with 1-2 metabolic syndrome components are still considered to have MHO in most previous studies) [5].
The possibility that there is a subset of people with obesity who are truly resistant to the adverse metabolic effects of excess body fat and weight gain is important because it provides a unique population to study the protective and pathogenic mechanisms involved in obesity-associated insulin resistance and metabolic diseases. Here, we present a unique case report that demonstrates MHO is not a myth.
Case Presentation
A 25-year-old woman with MHO participating in an ongoing, longitudinal study was evaluated in July 2016 and again in October 2021. In 2016, the participant was considered to have MHO based on normal: (1) fasting glucose (<100 mg/dL [5.6 mmol/L]), oral glucose tolerance (2-hour glucose